THE ANATOMY & MECHANICS OF A LITHIUM-ION BATTERY
What’s inside a lithium-ion battery? The battery components, how they function, and why these batteries are everywhere.
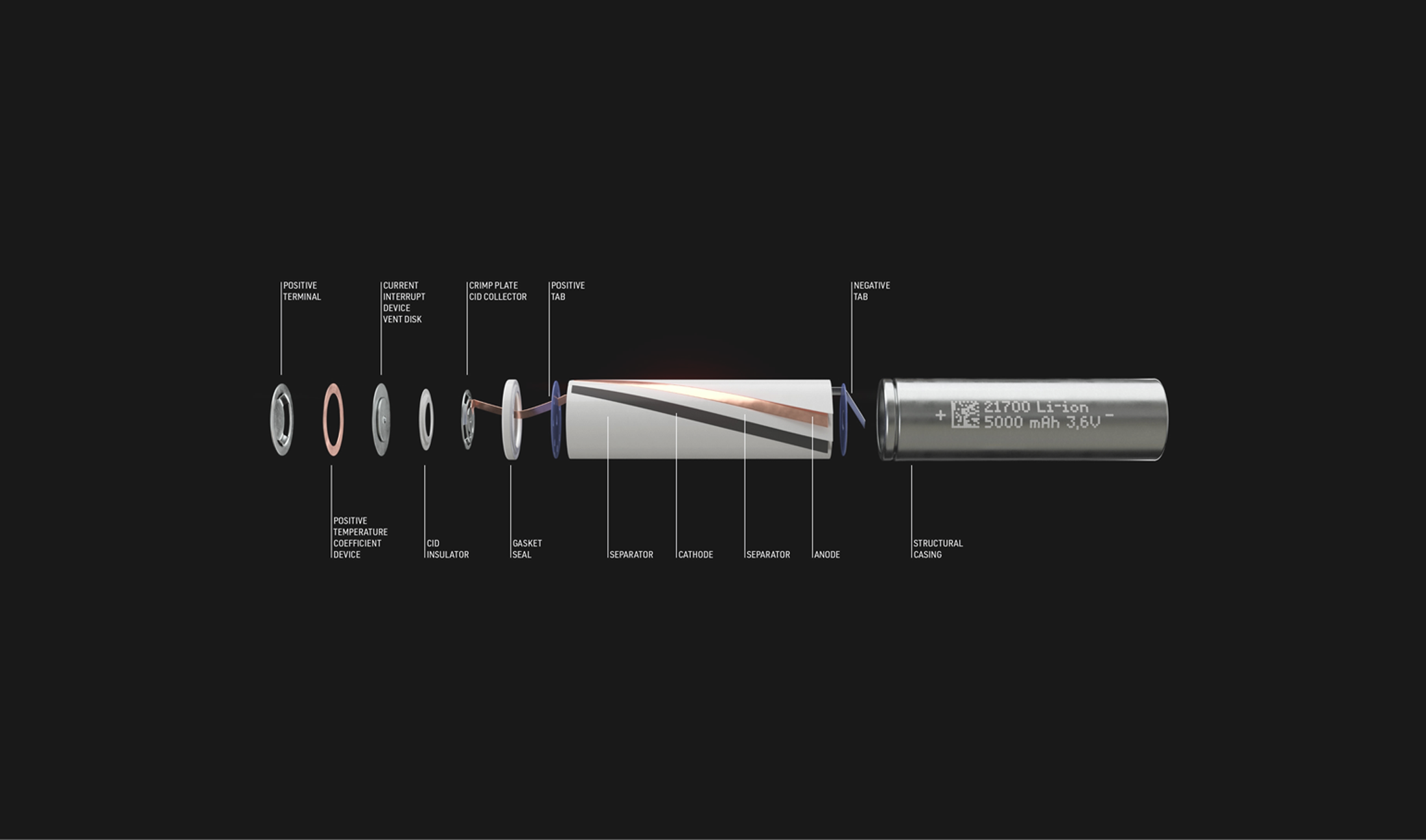
Battery components play a pivotal role as lithium-ion batteries silently integrate into our daily routines, energizing devices ranging from smartphones to electric vehicles. While their prevalence is undeniable, the inner workings of these rechargeable power sources often remain a mystery.
In this exploration, we’ll cut through the buzz and take a pragmatic look at the anatomy and mechanics of lithium-ion batteries—understanding what makes them tick and why they’ve become the go-to solution for our electronic devices. So, what exactly are lithium ions, what are the six lithium-ion battery components, how do they store and release energy, and what’s the fuss all about? Let’s break it down.
Why Lithium For Battery Components?
It probably goes without saying, lithium is the most fundamental element in a lithium-ion battery. Lithium is the lightest of all metals, has the greatest electrochemical potential, and provides the highest energy density. This trifecta makes it an unparalleled choice for various battery components.
What Are Lithium Ions?
As the lightest alkali metal, lithium possesses a single electron in its outermost shell. This lone electron makes lithium particularly eager to lose that electron and achieve a stable electron configuration. When lithium loses this outer electron, it becomes a lithium ion (Li+), carrying a positive charge. Put simply, lithium ions are small, charged particles that are used to generate electricity in batteries.
What’s Inside: Lithium-Ion Battery Components
A lithium-ion battery is comprised of six core battery components: anode, cathode, electrolyte, separator, current collectors, and the casing. In addition to these parts, there may be additional elements such as adhesives, insulation, and protective coatings, all depending on the design and application of the battery. The overall design and number of battery components can vary depending on the specific battery construction and application.
Anode
The anode is the negative electrode of the battery, which is made up of a porous carbon-based material, typically graphite. It serves as the starting point for the energy journey for the lithium ions and as a storage warehouse for the battery— storing the lithium ions when the battery is charged.
During discharge, the lithium ions move from the anode to the cathode. The movement of these ions creates free electrons in the anode, and these electrons create a charge. Conversely, charging the battery involves the transfer of lithium ions from the cathode back to the anode for storage. The anode is important because it helps the battery store energy efficiently and releases energy when needed to power your device.
Cathode
The cathode is the positively charged end of the battery; it determines the capacity and voltage of the battery and serves as the source of lithium ions for the chemical reactions that generate electrical energy. The battery’s capacity is determined by the cathode material. It is typically made of a metal oxide, such as lithium cobalt oxide, lithium manganese oxide, or lithium iron phosphate. The higher amount of lithium in the cathode, the higher the voltage of the battery.
The lithium ions move from the anode to the cathode when the battery is being used. When the cathode becomes full of lithium ions, the reaction stops and the battery is flat. Then we recharge our lithium-ion batteries again, and the external electric charge that we apply pushes the lithium ions back into the anode from the cathode.
The goal for the cathode material is to produce a reaction with a significantly higher (and more positive) standard potential than the anode material so that the electrons are attracted to the cathode. The difference in standard potential between the electrodes kind of equates to the force with which electrons will travel between the two electrodes. This is known as the cell’s overall electrochemical potential, and it determines the cell’s voltage. The greater the difference, the greater the electrochemical potential, and the higher the voltage.
Electrolyte
The electrolyte can be a liquid, gel or a solid substance, that acts as a medium for the flow of lithium ions between the anode and the cathode, allowing the battery to charge and discharge. During discharge, lithium ions move from the anode to the cathode through the electrolyte, generating an electric current.
The electrolyte allows for the efficient transport of lithium ions while blocking the flow of electrons directly between the cathode and anode. This selective conductivity ensures that the electrons follow an external circuit, creating the electric current that powers electronic devices. Its proper composition and characteristics are paramount for ensuring the efficiency, safety, and longevity of the battery, making it a pivotal component in the design and functionality of lithium-ion batteries.
Separator
The separator is a thin, porous membrane that separates the anode and cathode and prevents them from touching. If the anode and cathode were to touch, the battery could short-circuit and potentially cause a fire or explosion.
The separator allows the lithium ions to pass through it while blocking the flow of electrons, ensuring that the anode and cathode remain separate. Common materials used for separators include polyethylene, polypropylene, and ceramics. The porosity of the separator allows for the electrolyte to flow freely, which is important for maintaining the battery’s performance.
The separator must be carefully chosen and designed to ensure that it has the right properties, such as the appropriate thickness, porosity, and chemical stability. It must also be able to withstand the high temperatures and pressures that can be generated within the battery during use.
Current Collectors
Current collectors ensure the smooth flow of electrons between the electrodes and the external circuit. They greatly influencing the capacity, rate capability and long-term stability of lithium-ion batteries, making them essential contributors to overall battery performance. Common metals used for current collectors include aluminum for the cathode and copper for the anode. These materials are selected for their conductivity, durability, and compatibility with the electrochemical processes taking place within the battery.
Container (Casing)
The lithium-ion battery casing, often referred to as the battery enclosure or housing, is the protective outer structure that holds the internal components of a lithium-ion battery. Its primary purpose is to ensure the safety of the battery and its surroundings by containing and insulating the potentially volatile materials within. The casing is typically constructed from durable and heat-resistant materials to withstand the conditions inside the battery and potential external impacts. Materials commonly used include plastics, metals (such as aluminum or steel), and composite materials. The choice of material depends on factors such as the specific application, required durability, and safety considerations.

How Battery Components Drive The Function of a Lithium-Ion Battery
A lithium-ion battery operates by shuttling lithium ions back and forth between the anode and cathode through the electrolyte, with the flow of electrons controlled by the external circuit. This cyclic movement of ions and electrons generates electrical energy during discharge and allows for the recharging of the battery during the charging phase. This dynamic interplay between battery components also facilitates the recharging phase.
When a lithium-ion battery is charging, the lithium ions travel through the electrolyte from the cathode to the anode, where they are held until the battery is ready for use. This process is driven by a voltage difference between the anode and cathode, which is created by the chemical reactions that occur within the battery.
When the battery is discharged, the lithium ions travel through the electrolyte from the anode to the cathode, releasing energy as they go. This energy can be used to power a device, such as a smartphone or electric vehicle. The charging and discharging process of a lithium-ion battery is reversible, allowing the battery components to endure multiple charge and discharge cycles without a significant loss of capacity.
Why Lithium-Ion Batteries Are a Game Changer
So, what’s so special about lithium-ion batteries? Their main draw is their energy density—it’s around double that of a NiCad battery, meaning that a battery half the size will give the same amount of power. They’re light and compact which means they’re better for things like portable electronics than the heavy lead-acid batteries that start our gas cars. Lithium-ion batteries are preferred over other battery types for several reasons:
High energy density
Lithium-ion batteries possess an impressive capacity to store an abundance of energy in a relatively small area. These batteries are perfect for compact electronic gadgets, such as cell phones, laptops, and cars, where space is limited.
Lightweight
Lithium-ion batteries are lightweight compared to other battery types. Lightweight lithium and carbon-based materials are used for electrodes in these batteries. This makes them a popular choice for portable devices.
Low self-discharge
Lithium-ion batteries have a tendency to maintain their charge for a prolonged period of time when not in use due to their low rate of self-discharge. This makes them convenient for use in devices that are used infrequently, such as emergency backup power supplies.
Fast charging
Lithium-ion batteries can be charged quickly compared to other battery types, reducing the time needed to charge a device. They are capable of rapidly charging without risk of being damaged due to their exceptional efficiency and capacity to withstand high currents.
Long cycle life
Lithium-ion batteries can undergo many charge and discharge cycles before their performance begins to degrade, resulting in long cycle life. This makes them cost-effective in the long run since they need to be replaced less often than other battery types.
Eco-friendly
Lithium-ion batteries are more environmentally friendly than other battery types. They do not contain heavy metals like lead and cadmium, which can be harmful to the environment. Additionally, they can be recycled, reducing the amount of battery waste that ends up in landfills.
Overall, the combination of high energy density, lightweight, low self-discharge, fast charging, long cycle life, and eco-friendliness makes lithium-ion batteries the preferred choice for many applications. However, it is important to note that there is no perfect battery, and different battery chemistries may be more suitable for specific applications based on factors such as cost, safety, and performance requirements.
IntriPlex and Lithium-Ion Battery Components
Lithium-ion batteries have transformed the automotive industry and have become the go-to power source for electric vehicles. As a company manufacturing battery components and lid assemblies for EV batteries, we recognize the importance of producing high-quality and sustainable components to meet the growing demand for electric vehicles.
Our commitment to innovation and sustainability ensures that our parts not only meet but exceed industry standards, providing reliable and efficient performance while minimizing their impact on the environment. As the electric vehicle market continues to expand, we are excited to be playing a role in shaping the future of sustainable transportation through our contributions to the development of high-quality battery components and lid assemblies.
The role of quality testing and inspection in a metal stamping company cannot be overstated. It’s the final assurance that every component leaving their facility meets the highest standards. When considering a metal stamping partner, inquire about their quality control processes, as they are the guardians of reliability, consistency, and ultimately, your peace of mind.


